Overview
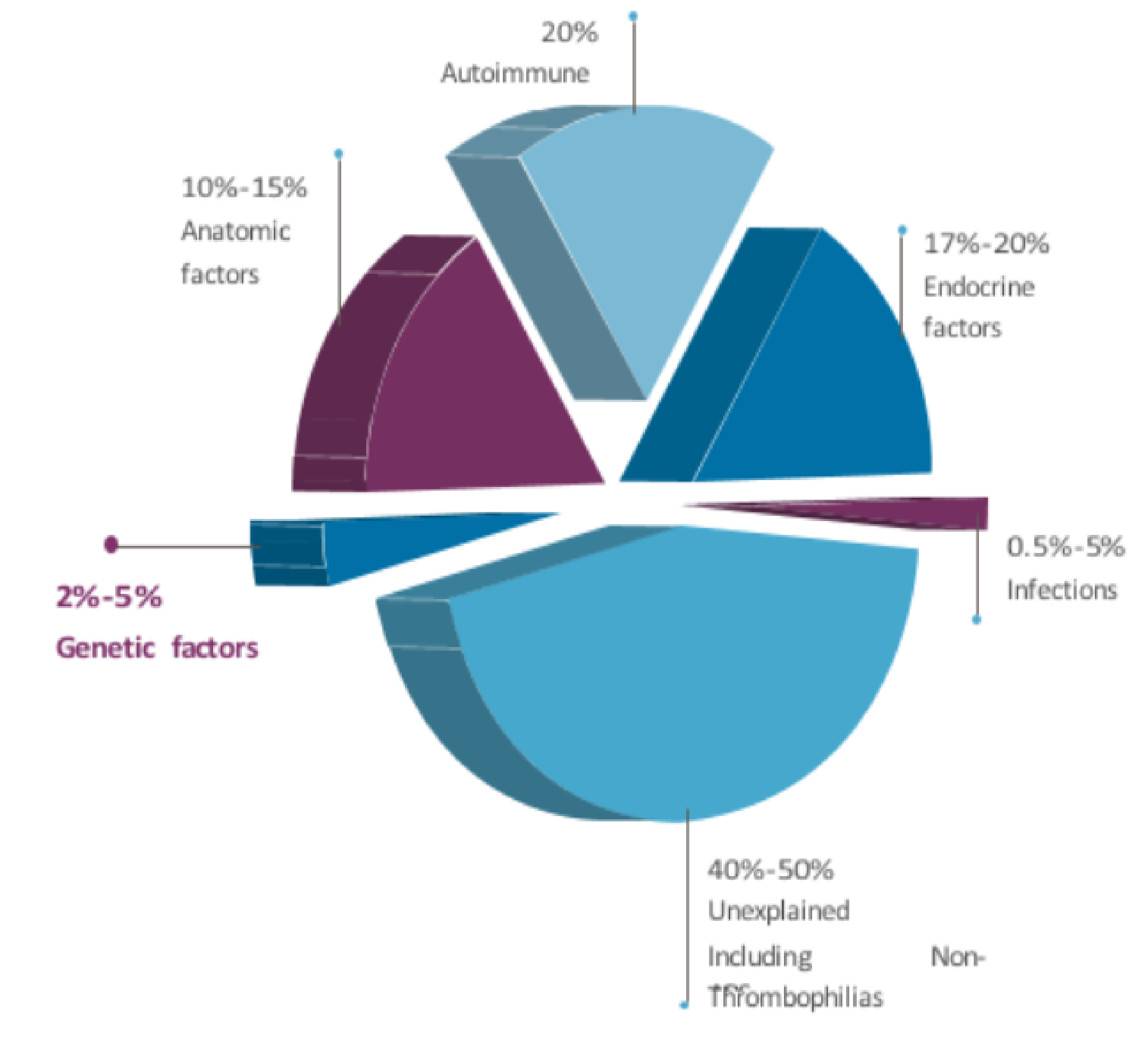
This 150-gene NGS panel facilitates comprehensive interrogation of genetic determinants of implantation failure and embryonic lethality. Targeted multidiscipline analysis encompasses thrombophilia, endocrine, structural, and receptor mutations across imprinting loci implicated in recurrent spontaneous abortion.
Methodology
Utilizing both germline gDNA and cfDNA from peripheral blood, our proprietary molecular inversion probe-based targeted sequencing platform achieves >1000X median coverage. Variant pathogenicity is determined by integrative evaluation against genome, transcriptome, and exome datasets using population-specific algorithms. CNV detection employs read-depth methodology.
POC testing refers to genetic analysis of fetal/embryonic tissue samples collected after a pregnancy loss (e.g. miscarriage). It can identify chromosomal causes like aneuploidy.
Women who experience recurrent pregnancy losses (two or more consecutive losses)
Losses at later gestational ages (>10 weeks)
Structural fetal abnormalities seen during the loss
Personal or family history suggestive of a genetic condition
Fetal tissue is collected by dilation and curettage after a loss. Tissue can be sent for karyotyping via microscopy to identify numeric chromosome changes. Alternatively, chromosomal microarray analysis (CMA) allows detecting smaller submicroscopic deletions/duplications.
CMA on POC samples provides a higher resolution of the fetal genome compared to karyotyping. It can detect abnormalities not seen on a karyotype that are causative in 5-10% of losses. CMA directly from tissue does not require viable cell culture.
Detects copy number variations (CNVs) down to 100kb in size
Provides information about recurrent CNVs and parental inheritance
Helps identify chromosomal causes undetected by standard karyotyping
Informs Recurrent Pregnancy Loss (RPL) workup, counseling, and management planning
Together, POC testing provides answers for recurring losses and guidance on recurrent risk in future conceptions. CMA improves diagnostic yield over karyotyping alone.
- Simultaneous assessment of maternal and fetal contributors to pregnancy wastage
- Integrates American Society for Reproductive Medicine criteria for etiologic evaluation
- Interrogates maternal thrombophilia, endocrine, and uterine anomalies
- Screens fetal aneuploidies, unbalanced rearrangements, segmental imbalances
- Informs personalized management algorithms for procreation success
This test is indicated for evaluation of idiopathic recurrent pregnancy loss defined as ≥2 consecutive miscarriages and recurrent implantation failure after IVF. Elucidation of underlying genetic susceptibilities allows tailored intervention to maximize livebirth potential in subsequent gestations.