Overview
Minagene offers comprehensive preimplantation genetic testing (PGT) utilizing next-generation sequencing and advanced bioinformatics. PGT screens embryos for aneuploidies (PGT-A), monogenic disorders (PGT-M), and structural rearrangements (PGT-S) to select the most euploid, mutation-free embryos for uterine transfer.
Methodology
Blastocyst biopsy on day 5 post-fertilization maximizes trophectoderm sampling without compromising development. Whole genome amplification and massively parallel sequencing precisely genotype all 24 chromosomes and interrogate pathogenic mutations at >20,000X coverage. Proprietary algorithms minimize artifacts to assure diagnostic accuracy.
Trophectoderm biopsy on day 5 maximizes both viable cell cohort and developmental synchrony for accurate results, safely avoiding risk to the inner cell mass. This enhances live birth rates better than earlier biopsies.
Why Screening is Critical
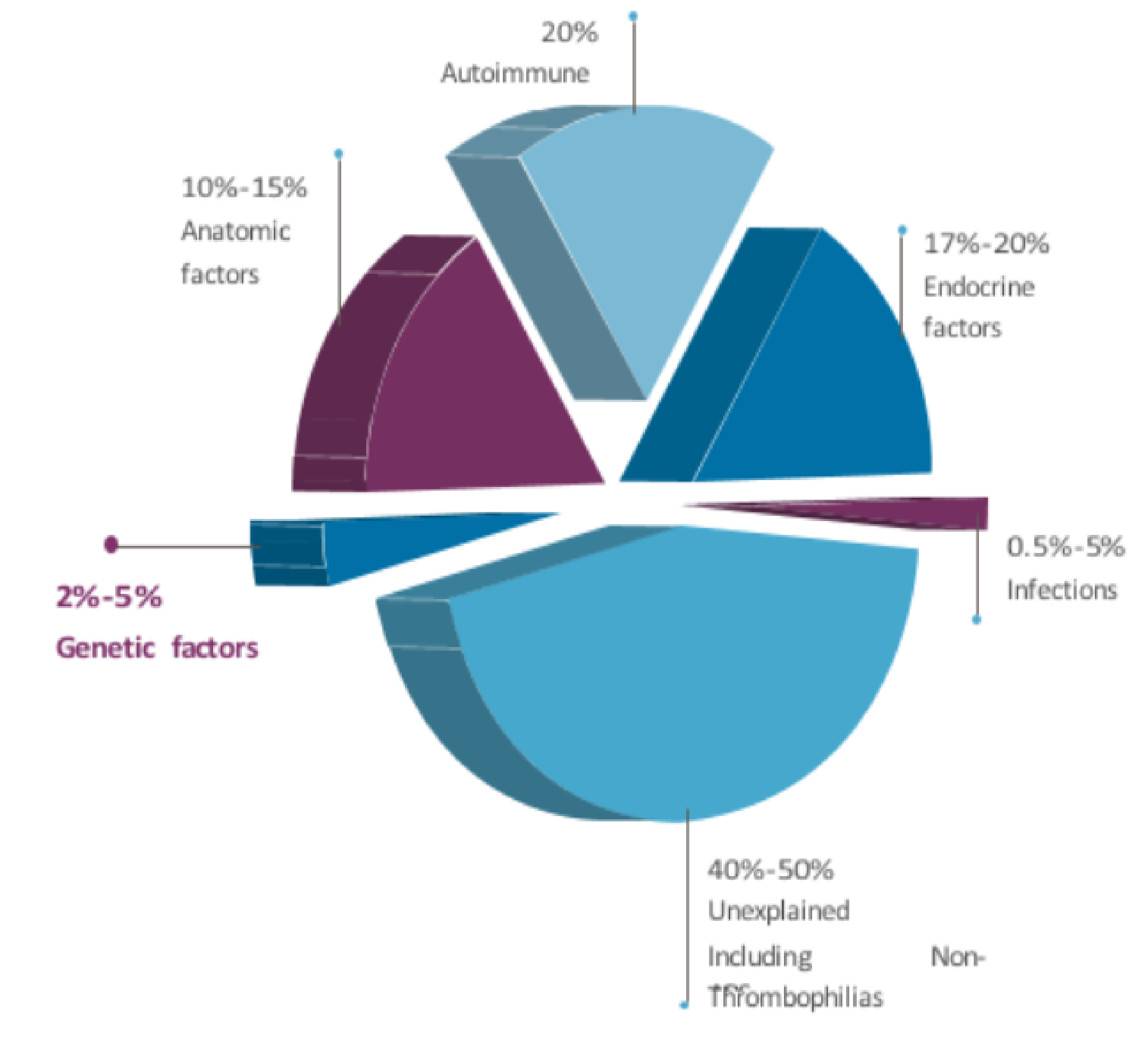
Aneuploidy is the leading cause of implantation failure and miscarriage. Without PGT, embryos transferred harbor an up to 75% risk of chromosome abnormalities undetectable by morphology alone. Screening identifies the healthiest embryos most likely to achieve live birth.
Why preimplantation genetic testing?
PGT optimizes ART success by selecting euploid embryos not carrying specific familial gene mutations for transfer. This alleviates repeated IVF cycles, miscarriages, termination of affected pregnancies, and liveborn offspring requiring lifelong care for genetic diseases.
Highlights of Minagene's preimplantation genetic testing (PGT)
Minagene’s PGT detects all 24 chromosome aneuploidies as well as over 1500 single gene disorders. It resolves structural variants and screens for unmatched maternal contamination with exact precision.
When to Consider Minagene’s preimplantation genetic testing (PGT)
Minagene’s preimplantation genetic
- Couples undergoing IVF
- Patients at any age who have had repeated implantation failure or recurrent pregnancy loss while undergoing IVF
- Women over 35 years old undergoing IVF
- Couples with recurrent miscarriages
- Positive history of chromosomal abnormalities in the family
- Diagnosed carriers of chromosomal aberrations