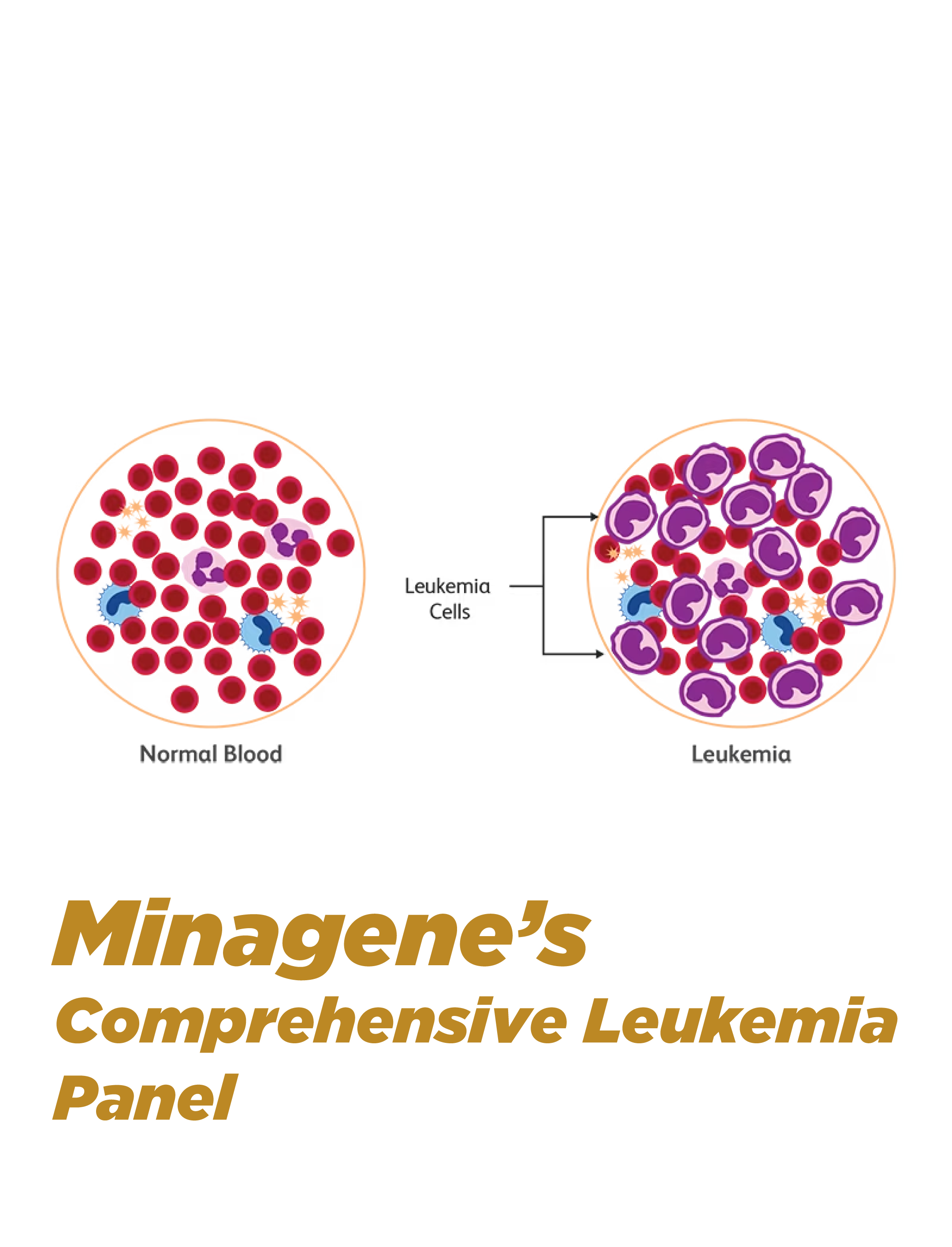
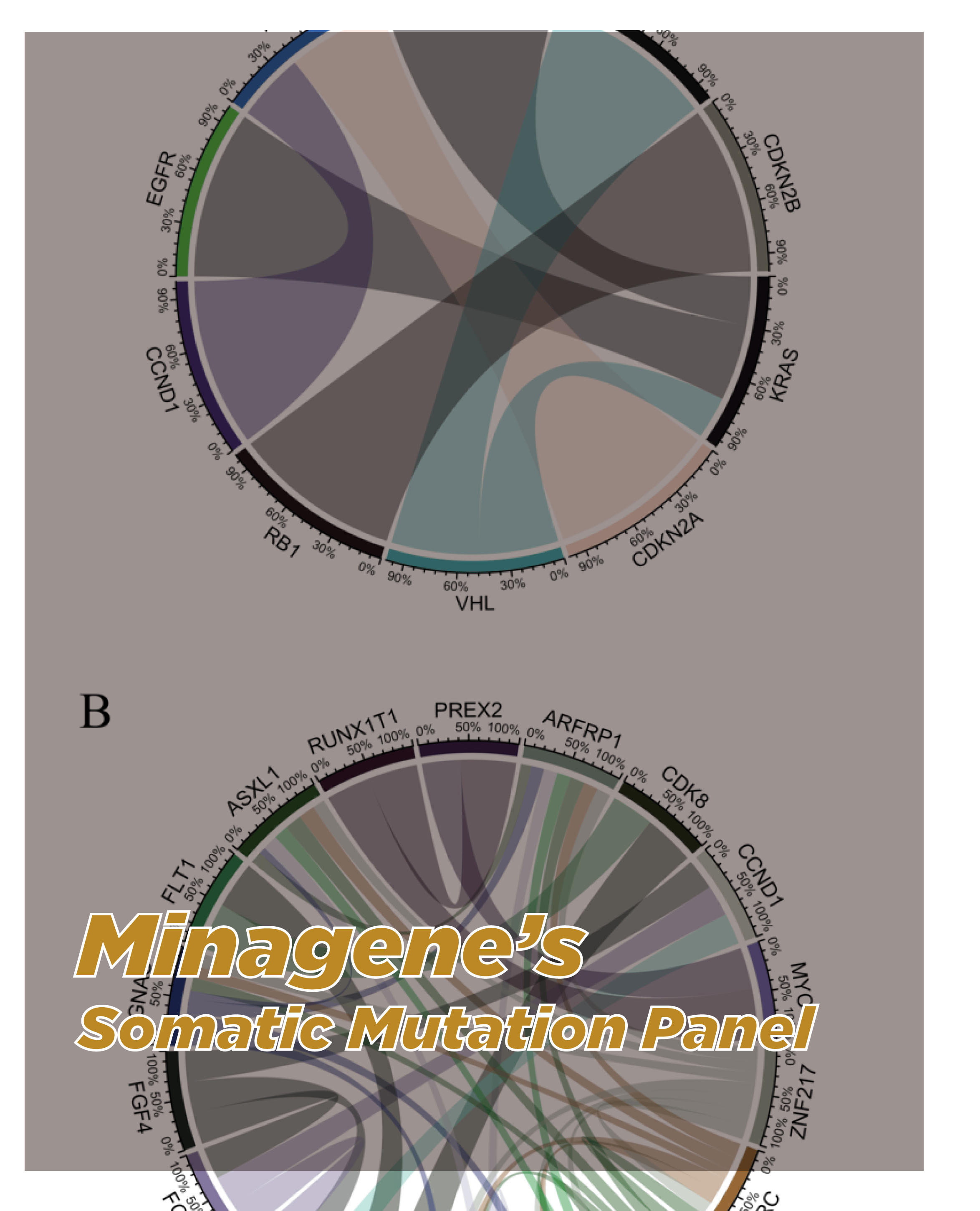
Overview
Minagene's Primary Immunodeficiency Gene Panel is a specialized genetic test designed to provide a comprehensive analysis of genetic mutations associated with primary immunodeficiency disorders. This advanced panel utilizes next-generation sequencing (NGS) technology to analyze a panel of genes involved in immune system function, allowing for precise diagnosis, personalized treatment strategies, and informed genetic counseling for individuals with primary immunodeficiency disorders.
Methodology
The Primary Immunodeficiency Gene Panel employs targeted NGS technology to sequence a panel of genes known to be associated with primary immunodeficiency disorders. The panel covers a wide range of genes involved in immune system development, function, and regulation. By analyzing these genes, the panel provides a comprehensive genetic profile that helps identify specific genetic mutations contributing to primary immunodeficiency disorders, guiding accurate diagnosis and personalized management.
Key Highlights of the Test:
-Comprehensive Genetic Evaluation: The Primary Immunodeficiency Gene Panel offers a comprehensive evaluation of genetic mutations associated with primary immunodeficiency disorders. It analyzes key genes involved in immune system function and regulation, including immunoglobulin genes, cytokine receptors, and genes associated with T and B cell development. This comprehensive approach enables the identification of potential causative genetic mutations, aiding in accurate primary immunodeficiency disorder diagnosis.
-Precise Diagnosis: By identifying specific genetic mutations, the panel facilitates precise diagnosis and differentiation between different subtypes of primary immunodeficiency disorders. It helps categorize disorders such as common variable immunodeficiency (CVID), severe combined immunodeficiency (SCID), and selective antibody deficiencies, allowing for more targeted management strategies.
-Personalized Treatment Selection: The panel’s genetic insights assist in personalized treatment selection for individuals with primary immunodeficiency disorders. By identifying specific genetic mutations, the panel helps guide treatment decisions, including immunoglobulin replacement therapy, hematopoietic stem cell transplantation (HSCT), targeted therapies, or gene therapy based on the underlying genetic abnormalities.
-Prognostic Assessment: The Primary Immunodeficiency Gene Panel provides valuable prognostic information by identifying genetic mutations associated with disease severity and clinical outcomes. This information aids in risk stratification and treatment planning, allowing clinicians to optimize therapeutic interventions and improve patient prognosis.
-Genetic Counseling and Family Screening: The genetic information obtained from the panel can be used for genetic counseling and family screening. It helps identify family members who may carry pathogenic mutations associated with primary immunodeficiency disorders, enabling early detection and intervention in at-risk individuals.
-Diagnostic Evaluation: The Primary Immunodeficiency Gene Panel is recommended for individuals suspected of having primary immunodeficiency disorders, particularly in cases where there is a history of recurrent, severe, or unusual infections. The panel aids in precise diagnosis and differentiation between different subtypes of primary immunodeficiency disorders.
-Personalized Treatment Planning: The test is valuable for personalized treatment planning in individuals with primary immunodeficiency disorders. By identifying specific genetic mutations, the panel helps guide treatment decisions, including the selection of appropriate immunoglobulin replacement therapies, HSCT, targeted therapies, or gene therapy based on the underlying genetic abnormalities.
-Prognostic Assessment: The panel’s genetic insights assist in prognostic assessment by identifying genetic mutations associated with disease severity and clinical outcomes. This information aids in risk stratification and treatment planning, enabling clinicians to optimize therapeutic interventions and improve patient prognosis.
-Genetic Counseling and Family Screening: The panel’s results can be used for genetic counseling and family screening. It helps identify family members who may carry pathogenic mutations associated with primary immunodeficiency disorders, facilitating early detection and intervention in at-risk individuals.
-Research and Clinical Trials: The Primary Immunodeficiency Gene Panel can be utilized in research studies and clinical trials focusing on primary immunodeficiency disorders. The panel’s comprehensive genetic profiling may contribute to a better understanding of the genetic basis of these disorders and help identify potential therapeutic targets.