Overview
Minagene's Clinical Exome V4 is an advanced genetic testing service that utilizes state-of-the-art sequencing technologies to analyze the protein-coding regions of the genome, known as the exome. By examining thousands of genes associated with various genetic disorders, this comprehensive test provides clinicians with valuable insights into the molecular basis of complex and rare diseases, enabling accurate diagnosis, personalized treatment strategies, and improved patient care.
Methodology
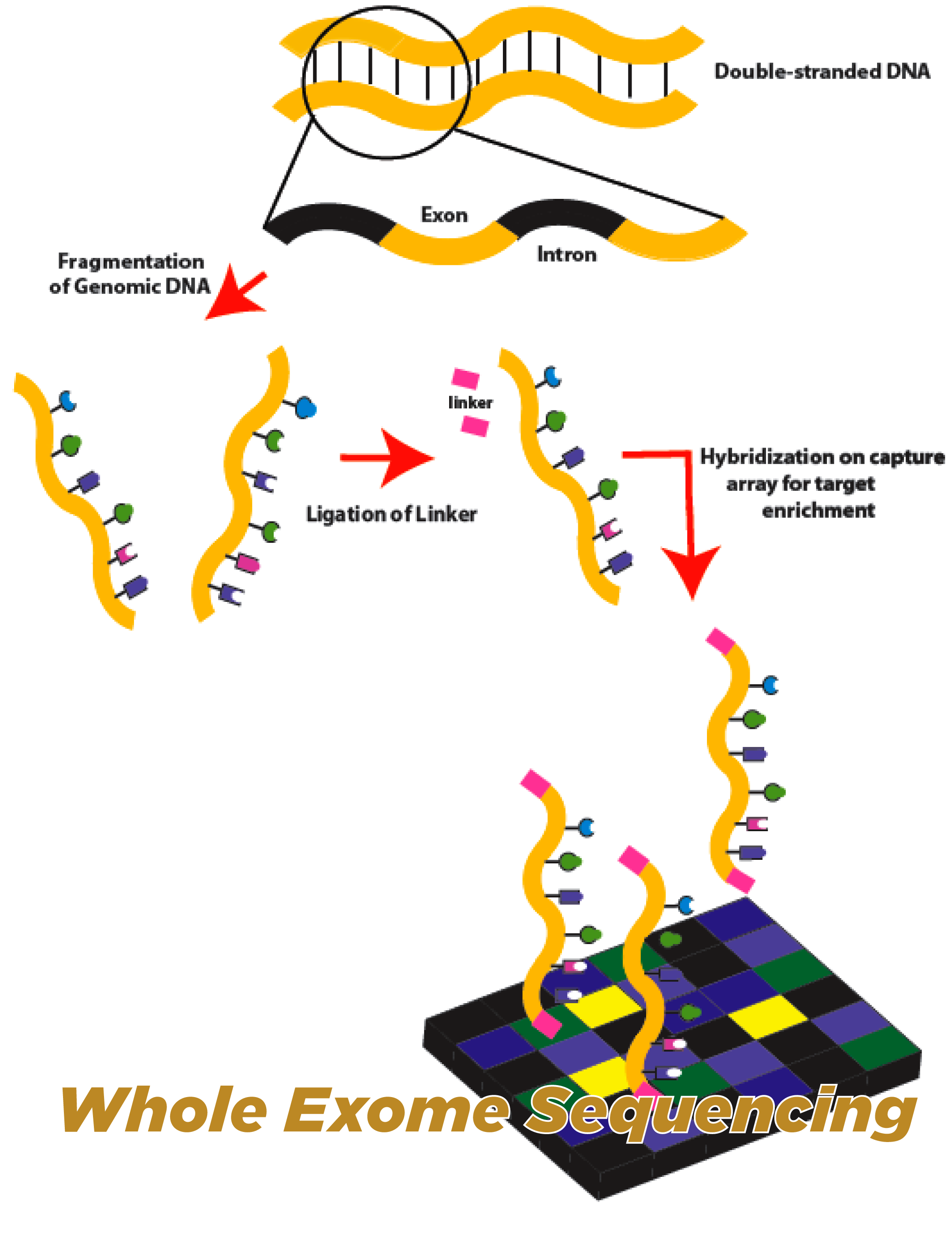
The Clinical Exome V4 test employs next-generation sequencing techniques to capture and sequence the exonic regions of an individual’s genome. This high-throughput approach allows for the analysis of thousands of genes simultaneously, providing a broad and in-depth assessment of the genetic variants that may contribute to a patient’s condition.
Key Highlights of the Test:
Comprehensive Genetic Analysis: The Clinical Exome V4 test offers a comprehensive analysis of the exome, covering a vast number of disease-associated genes. By examining a wide range of genetic variants, including single nucleotide variants (SNVs), small insertions and deletions (indels), and copy number variations (CNVs), the test provides a comprehensive view of the patient’s genetic landscape, aiding in the identification of disease-causing variants.
Accurate Diagnosis: The test enables accurate diagnosis by identifying pathogenic or likely pathogenic variants associated with genetic disorders. By comparing the patient’s genetic profile with a vast database of known disease-associated variants, the test helps clinicians pinpoint the underlying cause of the patient’s condition, leading to more precise diagnoses and appropriate treatment plans.
Personalized Treatment Strategies: The Clinical Exome V4 test assists in developing personalized treatment strategies by identifying genetic variants that may impact drug response or guide targeted therapies. By understanding an individual’s unique genetic makeup, clinicians can tailor treatment plans, select appropriate medications, and optimize therapeutic outcomes.
Identification of Rare and Novel Variants: The test has a particular focus on detecting rare and novel variants that may not be captured by other genetic testing methods. By exploring the exome comprehensively, it enhances the chances of identifying unique genetic variants that may be responsible for a patient’s condition, even in cases where a diagnosis was previously elusive.
Enhanced Variant Interpretation: Minagene’s Clinical Exome V4 benefits from advanced variant interpretation algorithms and a curated variant knowledgebase. This enables clinicians to access up-to-date information on variant pathogenicity, supporting accurate variant classification and reducing the risk of misinterpretation.
Complex and Undiagnosed Cases: The Clinical Exome V4 test is particularly valuable in complex and undiagnosed cases where the underlying cause of a patient’s condition is unclear. By conducting a comprehensive analysis of the exome, it increases the chances of identifying disease-causing variants and providing a definitive diagnosis.
Rare Genetic Disorders: The test is beneficial in cases suspected to have rare genetic disorders. It enables the detection of rare and novel variants associated with these conditions, aiding in accurate diagnosis, appropriate management, and genetic counseling for patients and their families.
Therapeutic Decision-Making: The test assists in therapeutic decision-making by identifying genetic variants that may impact drug response or guide targeted therapies. This information helps clinicians select the most suitable medications, optimize treatment plans, and improve patient outcomes.
Family Screening and Counseling: The Clinical Exome V4 test supports family screening and counseling for genetic disorders. By identifying disease-causing variants, it allows for the identification of at-risk family members and facilitates informed decision-making regarding family planning and genetic counseling.
Research and Clinical Trials: The test contributes to research efforts and clinical trials focused on genetic disorders. By participating in genetic studies, patients can help advance knowledge about rare diseases, potentially leading to the development of new treatments and therapeutic approaches.